Reaction of Cyclohexene With Bromine
Add additional sketchers using the drop- down menu in the bottom right corner. The majority of examples feature only simple cyclic alkene substrates such as cyclopentene and cyclohexene.

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
Explain the formation of major and minor products by reference to the relative stabilities of primary secondary and tertiary carbocation intermediates.

. Reports of enantioselective reactivity retain this limited substrate scope and are characterized by poor catalytic efficiency that can result in multiday reaction times 26 27. Reaction of bromine water with cyclohexene. Chelated reduction zinc borohydride.
Figure 104e Formation of Halohydrin Figure 104f Mechanism. The reaction takes place at room temperature if the reactants are in the gaseous state ethene. Two general types of monoalkenes are distinguished.
In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or. However since H 2 O is the solvent its concentration is much higher than that of Br so the major. Diels-Alder reaction will occur that forms a cyclohexene.
Draw one structure per sketcher. To make polyamide 6 pure cyclohexanone is required. This is more energy efficient than the other processes.
BrO-Bromine monoxide anion BrO Bromine monoxide cation C v. Alkynes under metal catalysts for example cobalt can also go under cycloaddition reaction called alkyne trimerization. The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene are shown in the green box.
Also called α-olefins terminal alkenes are more useful. Felkin-Anh reduction LiAlH 4. Cyclohexene epoxide opening axial.
Intramolecular carbonyl ene reaction. Do not breathe the vapours. Want to see the full answer.
Consider the reaction of bromine with the pictured alkene. Chelate Cram Addition MeMgBr. Colour of the bromine water solution is decolourized as it reacts with ethene.
NaBr-sodium bromide anion NaBr sodium bromide cation C v. SiBr Silicon. Cyclohexene epoxide opening axial.
Students should be able to. Intramolecular carbonyl ene reaction. One of the main pillars of our policy is to provide our customers with the highest quality products at.
Keep bromine away from your skin. Use only in the FUME HOOD. When the mixed oil is heated under pressure with copperll and chromiumlll oxides the.
Most are made from petroleum. Cyclohexanol can be irritating to the respiratory system and skin. Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5.
Such reaction is highly selective in stereochemistry. You do not have to consider stereochemistry. The ICSC project is a common undertaking between the World.
H H H H H wireframe labels Draw the organic products of reaction of the alkyne above with H3O in the presence of HgSO4. Aldol Reaction syn product. Science Chemistry QA Library Consider the reaction of bromine with the pictured alkene.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The main target users are workers and those responsible for occupational safety and health. Consider the reaction of bromine with the pictured alkene.
The formation of major and minor products in addition reactions of unsymmetrical alkenes. Our modern society is based to a large degree on the chemicals we discuss in this chapter. Aldol Reaction anti product.
Aldol Reaction syn product. A demonstration of bromine substitution and addition reactions is helpful at this point. But liquid alkenes like cyclohexene react with bromine water solution in the.
O undesirable effect means an adverse reaction for human health attributable to the normal or reasonably foreseeable use of a cosmetic product. Bromine causes severe burns. Do not breathe vapours and prevent contact with skin.
Extensive R D activities are carried out to ensure that our formulations are both effective and of consistent high quality. Cyclohexene has an. Stereoselective Aldol Reaction Cis gives Syn.
Alkene is often used as synonym of olefin that is any hydrocarbon containing one or more double bonds. The reaction is regioselective leading to Markovnikov-oriented products and the halofluorinated adducts follow anti-addition in the case of cyclohexene and 1-methylcyclohexene. A synthetically useful radical-relay CH oxidation.
Outline the mechanisms for these reactions. Chelated reduction zinc borohydride. A more recent route to cyclohexanol is the Asahi process from benzene via its hydrogenation to cyclohexene and subsequent hydration to alcohol.
Check out a sample. R M is the supplier of chemicals marketed under the label R M Chemical with the aim of providing the most comprehensive range of chemical products. Chelate Cram Addition MeMgBr.
In the second step of the mechanism both H 2 O solvent and Br produced in the first step are nucleophiles and have the chance to react with the cyclic bromonium ion. In organic chemistry an alkene is a hydrocarbon containing a carboncarbon double bond. Felkin-Anh reduction LiAlH 4.
Aldol Reaction anti product. Esteves Synthesis 2010 2379-2382. P serious undesirable effect means an undesirable effect which results in temporary or permanent functional incapacity disability hospitalisation congenital anomalies or an immediate vital risk or death.
The reaction equation for bromine addition of ethene for example is. Citation neededHowever the International Union of. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The use of bromine to test for unsaturation. Three alkynes goes under a 222 cyclization reaction and rapidly join to form a. Potassium permanganate is a strong oxidizing agent.
Stereoselective Aldol Reaction Cis gives Syn. BrF-bromine fluoride anion BrF bromine fluoride cation C v.
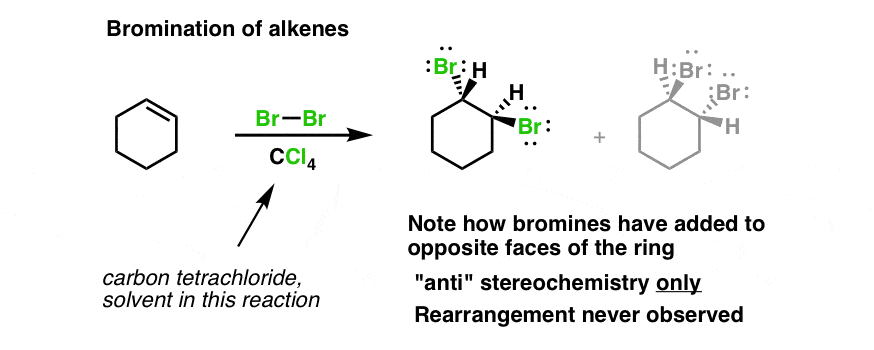
Bromination Of Alkenes Master Organic Chemistry
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Bromination Of Cyclohexene Under Conditions Given Below Yields Ltimg Src Https D10lpgp6xz60nq Youtube

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram
Comments
Post a Comment